Our Research
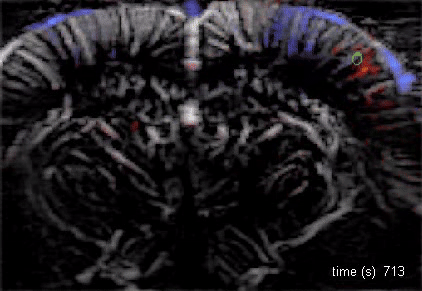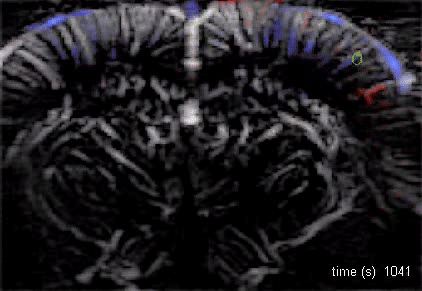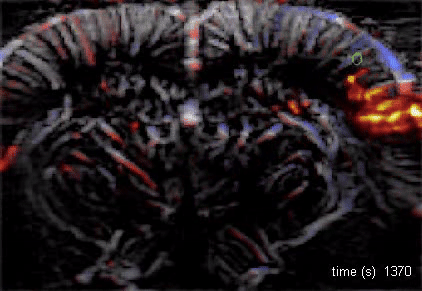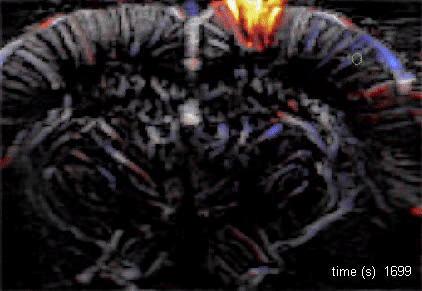
The Macé lab addresses how brain-wide networks interact to produce a particular behavior. We use functional ultrasound imaging, a technique we pioneered, to follow neuronal activity in the whole mouse brain. We combine this technique with targeted genetic circuit manipulations and electrophysiological measurements. One goal is to better understand how cognitive processes, such as behavioral switching, arise in the brain across multiple scales and uncover dysfunctions of these networks in psychiatric disorders. Our lab also studies visual processing and develops innovative optogenetic strategies against blindness to restore visual perception and visuomotor function.
Latest Publications
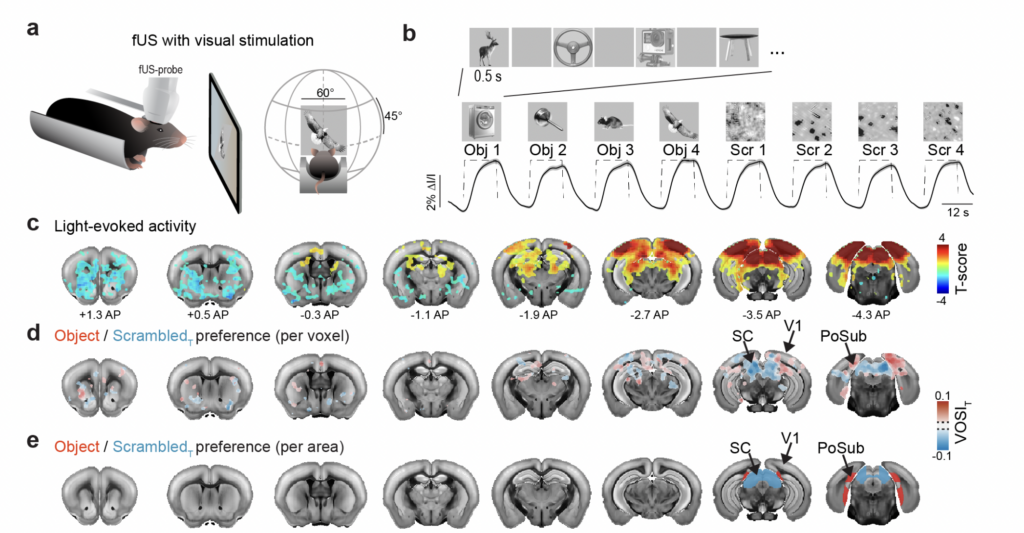
Visual objects refine head direction coding
Science, 2025
Using functional ultrasound (fUS) imaging in mice, we conducted a brain-wide screen and identified brain areas that were preferentially activated by images of objects compared to scrambled versions of the same stimuli. While visual cortical areas did not show a significant preference, regions associated with spatial navigation were preferentially activated by visual objects. Electrophysiological recordings in the postsubiculum, the primary cortical area of the head direction (HD) system, further confirmed a preference for visual objects, which was present in both HD cells and fast-spiking interneurons. Finally, we found that visual objects dynamically modulate HD cells, selectively increasing firing rates for HD cells aligned with a visual landmark’s direction, while suppressing activity in HD cells coding for other directions. These results reveal that visual objects refine population-level coding of head direction.
Efficient and sustained optogenetic control of nervous and cardiac systems
Nature Biomedical Engineering, 2025
Here we report ChReef, an improved variant of the channelrhodopsin ChRmine. ChReef offers minimal photocurrent desensitization, a unitary conductance of 80 fS and closing kinetics of 30 ms, which together enable reliable optogenetic control of cells at low light levels with good temporal fidelity and sustained stimulation. We demonstrate efficient and reliable red-light pacing and depolarization block of ChReef-expressing cardiomyocyte clusters. We used adeno-associated-virus-based gene transfer to express ChReef in retinal ganglion cells, where it restores visual function in blind mice with light sources as weak as an iPad screen. Toward optogenetic hearing restoration, ChReef enables stimulation of the auditory pathway in rodents and non-human primates with nanojoule thresholds, enabling efficient and frequency-specific stimulation by LED-based optical cochlear implants.
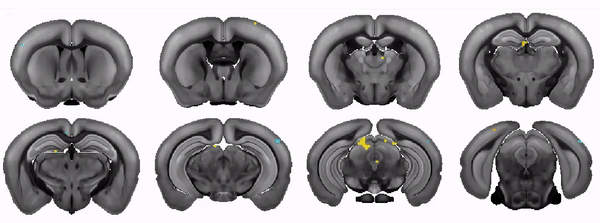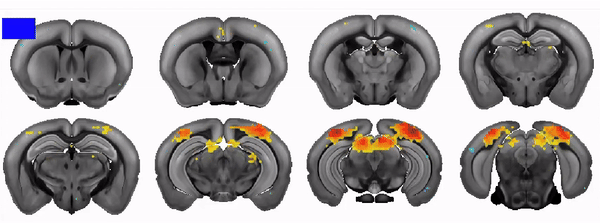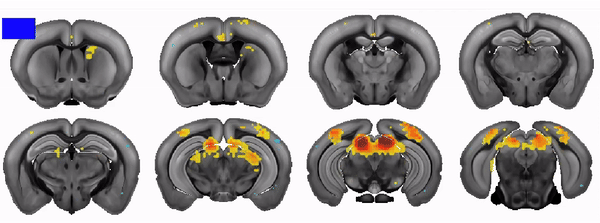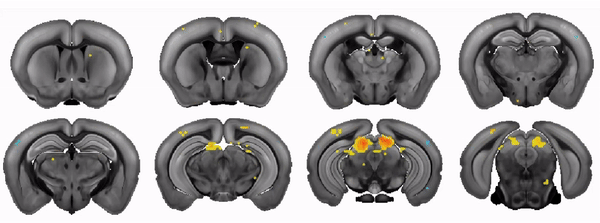
The COMBO window: A chronic implant for multiscale circuit interrogation in mice
PLOS Biology, 2024
Neuroscientists studying the neural correlates of mouse behavior often lack access to the brain-wide activity patterns elicited during a specific task of interest. Fortunately, large-scale imaging is becoming increasingly accessible thanks to modalities such as Ca2+ imaging and functional ultrasound (fUS). However, these and other techniques often involve challenging cranial window procedures and are difficult to combine with other neuroscience tools. We address this need with an open-source 3D-printable cranial implant—the COMBO (ChrOnic Multimodal imaging and Behavioral Observation) window. The COMBO window enables chronic imaging of large portions of the brain in head-fixed mice while preserving orofacial movements. We validate the COMBO window stability using both brain-wide fUS and multisite two-photon imaging. Moreover, we demonstrate how the COMBO window facilitates the combination of optogenetics, fUS, and electrophysiology in the same animals to study the effects of circuit perturbations at both the brain-wide and single-neuron level. Overall, the COMBO window provides a versatile solution for performing multimodal brain recordings in head-fixed mice.